Research Overview
DNA repair abnormalities in cancer
Defects in DNA repair are present in many cancers and likely play a fundamental role in tumorigenesis. Our laboratory investigates the role of DNA repair defects in cancer, with a focus on how disruption of BRCA1-dependent DNA repair pathways affects normal DNA repair choice, and leads to both genomic instability and epigenetic instability. Our overall aim is to characterize DNA repair defects in individual cancers and develelop specific therapeutic strategies that exploit these defects.
Identifying genomic mechanisms of exceptional response and acquired resistance to targeted therapy
We apply current NGS sequencing methods to identify mechanisms of exceptional response to novel agents employed in clincal trials at Rutgers Cancer Institute as well as explore mechanisms of acquired resistance to targeted therapeutics. This is achieved through direct analysis of patient tissues and development of novel cancer models.
Identifying novel mechanisms of response and resistance to cancer immunotherapy
Ongoing DNA repair defects may also lead to tumor immunogenicity and vulnerability to immune checkpoint blockade. Through both laboratory and computational approaches, we are investigationg potential mechanisms of response and resistance to immune checkpoint blockade.
Our laboratory uses cutting-edge methods in molecular and cell biology applied to cell and animal cancer models as well as analysis of primary human cancer specimens. We work closely with our colleagues in computational biology and clinical trials research in many of our projects.
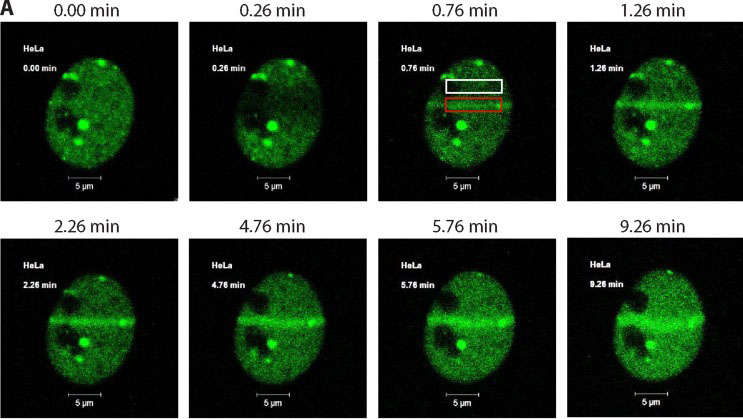
Cells were transfected with a plasmid expressing a p200 CUX1-GFP fusion protein.
DNA was damaged using 351/364 nm laser micro-irradiation and images were acquired immediately before DNA damage and periodically thereafter using the Argon laser (488 nm).
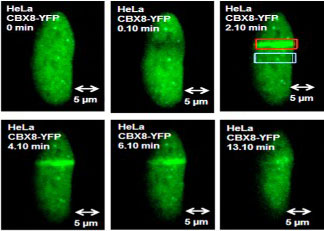
HeLa cells were plated as in A and under similar experimental condition; DNA was damaged using the UV laser (500 iterations in a narrow rectangular region) of Zeiss LSM 510 Meta confocal microscope at 63× water immersion. The images were captured right before and after damage and subsequently at 1-min intervals.

