Research Overview
The Molecular Cancer Prevention Laboratory, led by Dr. Xiyuan Zhang at the Rutgers Cancer Institute, leverages genome-wide sequencing technologies to decipher the etiology and progression of pediatric sarcomas and cancer predisposition syndromes, particularly neurofibromatosis type 1 (NF1). Our work is dedicated to enhancing the diagnostic, preventive, and therapeutic utilities of molecular profiling, aiming to improve our understanding of cancer biology and benefit our patients with targeted therapies.
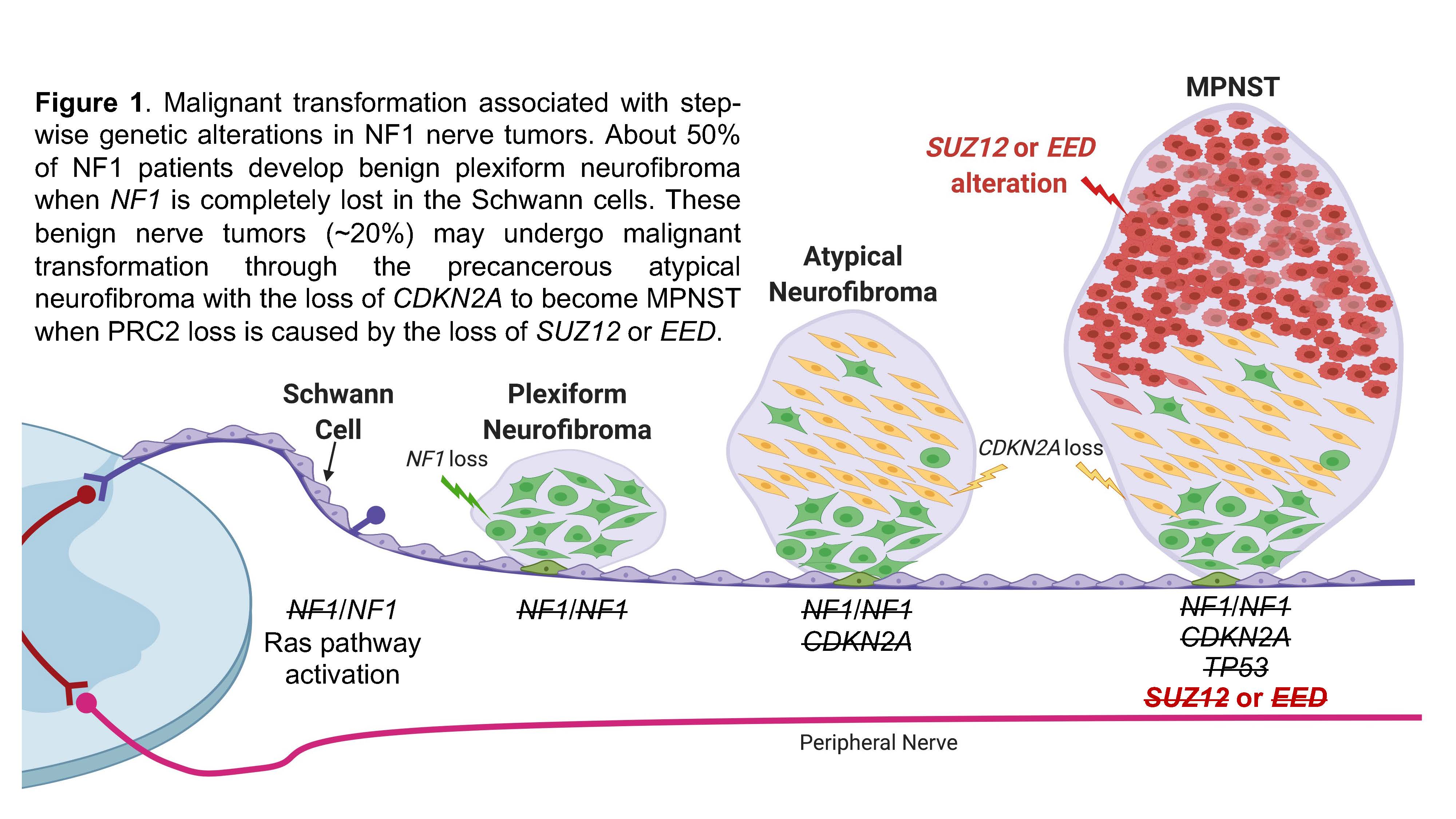
Lifetime risk of malignant transformations
Patients with a cancer predisposition syndrome (CPS) have increased risk of developing cancer compared to the general population. Neurofibromatosis type 1 (NF1) is the most common CPS, which is caused by inactivating mutations in the tumor suppressor gene NF1: ~1/3000 newborns are born with a germline mutation in NF1, which shows 100% penetrance in adults. A life-threatening complication associated with NF1 is that one may develop aggressive, therapy-resistant, and highly metastatic malignant peripheral nerve sheath tumors (MPNST, a sarcoma). Carriers of NF1 have a 1000 times higher lifetime risk of developing MPNST than the general population. This cancer is the product of a process called malignant transformation, in which plexiform neurofibroma (PN, a benign tumor) progresses through a precancerous stage called atypical neurofibroma (AN, a premalignant tumor) with an accompanying oncogenic activation. About 10% of NF1 patients develop MPNST as the result of this process, which is associated with step-wise genetic alterations (Figure 1, Zhang et al. Genes, 2020). While PN alone can be effectively treated by the FDA-approved selumetinib and mirdametinib, MPNST currently has no effective therapy besides complete surgical removal with wide negative margin, which is often difficult due to the location of the tumor and the complex involvement of nerves. It is therefore critical to prevent MPNST development through early detection of PN and AN that are at risk of undergoing malignant transformation. It is also essential to identify effective targeted therapy to treat this aggressive disease. Research in our laboratory focuses on understanding and modeling NF1 disease progression and identifying unique vulnerabilities to be targeted in preclinical studies.
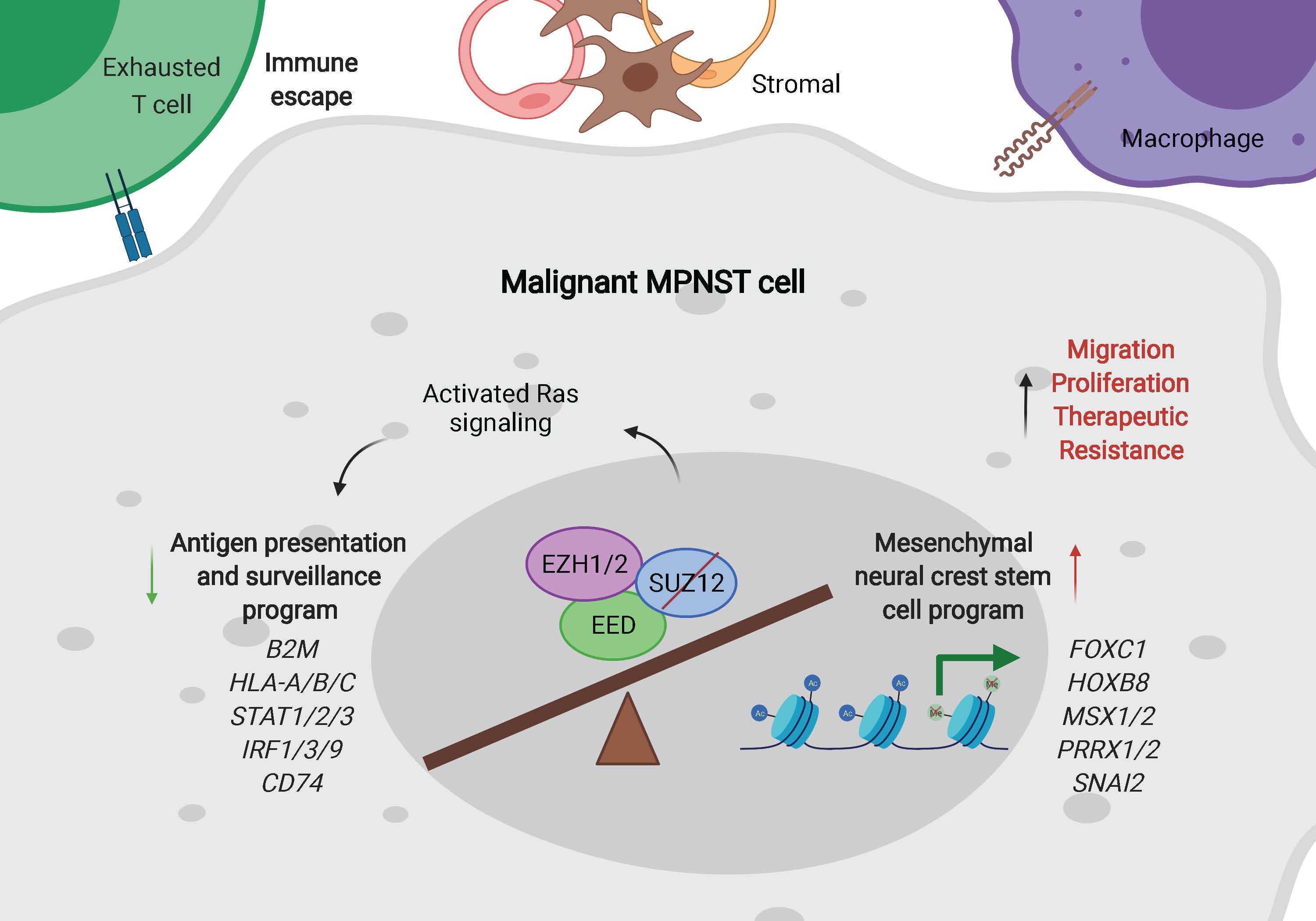
Epigenetic alterations in MPNST
Recurrent mutations in SUZ12 and/or EED, two key components of the polycomb repressive complex 2 (PRC2), lead to loss of tri-methylation of histone H3 lysine 27 (H3K27me3) in more than 80% of MPNST. PRC2 and its product H3K27me3 are critical epigenetic modifiers that contribute to transcriptional repression of genes essential for normal cellular function and organismal development. Our previous work coupling human MPNST model systems and single-cell RNA sequencing (scRNAseq) in patient samples characterized the epigenetic consequences of PRC2 loss, suggesting that 1) MPNST demonstrates a de-differentiated cellular phenotype that deviates from its cell-of-origin, Schwann cells; 2) the identification of at-risk AN provides a critical window for the prevention and early diagnosis of MPNST; and 3) epigenetic approaches may be utilized for efficient prevention of MPNST and its potential metastasis (Figure 2, Zhang et al. Cell Reports, 2022). However, the lack of proper preclinical models is a major barrier in addressing these problems.
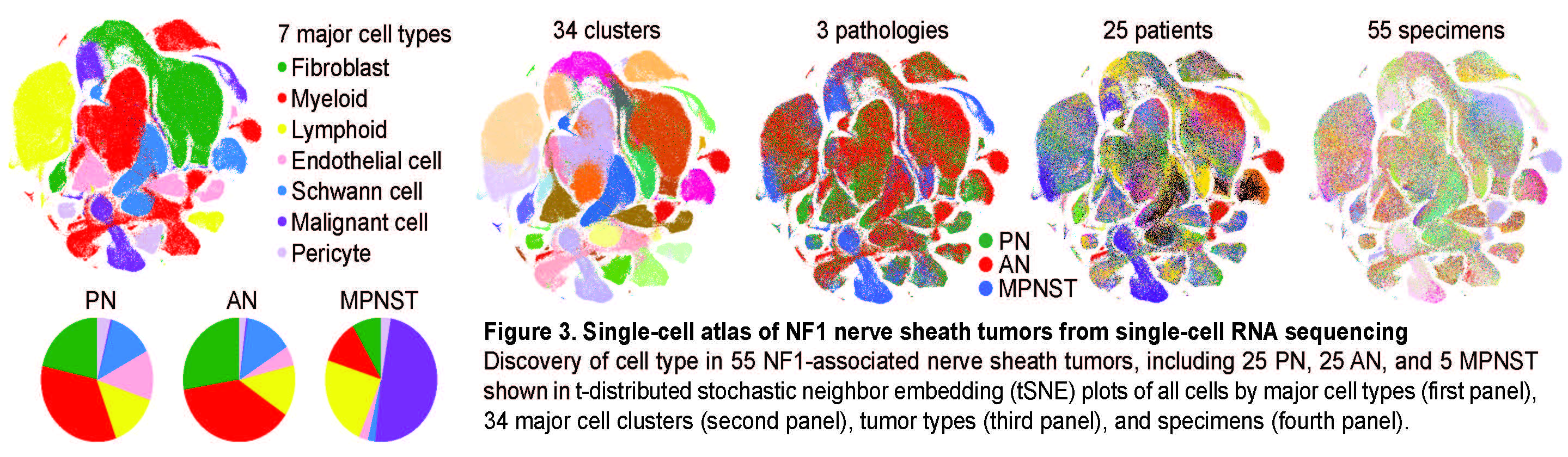
Intratumoral heterogeneity in NF1-associated nerve sheath tumors
To understand the molecular and transcriptional changes that occur during the malignant transformation of NF1 tumors, we built an NF1 tumor single-cell atlas. Using scRNAseq, we profiled the intra-tumoral heterogeneity of clinically annotated NF1 nerve sheath tumors collected from all pathological stages to 1) dissect the oncogenic mechanisms of NF1-deficient Schwann cells during the malignant transformation and 2) to describe the concurrent changes in the tumor microenvironment (Figure 3). Importantly, we discovered a unique “transitioning” cell population in some AN that marked them as potentially high risk for malignant transformation. We used the unique transcriptomic signatures of these cells as biomarkers in a clinical tool for early identification of MPNST, as they facilitate the early detection of high risk AN and prevention of MPNST.
Research in the Zhang Laboratory aims to address mechanisms underlying the malignant transformation of NF1-associated nerve sheath tumors through ongoing research projects:
-
Identification of epigenetic modulators that both positively and negatively regulate the activation of oncogenes during the malignant transformation of NF1 tumors.
-
Characterization of gene expression changes over the course of tumor progression using patient samples and tumor-derived cell lines.
-
Evaluation of the role that extra chromosomal DNA (ecDNA) plays in driving malignant transformation in high-risk PN and AN.

