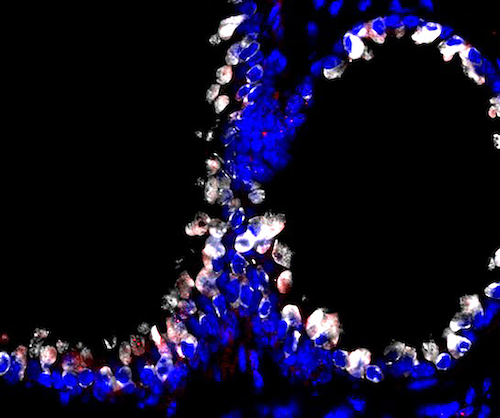
Confocal microscopy image showing that targeted phage particles (red) bind specifically to lung cells (club cell secretory protein in white and nucleus in blue) when administered via aerosol in animal models.
New Brunswick, N.J., December 10, 2020 – Investigators at Rutgers Cancer Institute of New Jersey and Rutgers New Jersey Medical School recently identified a new method for safe and effective delivery of medicines to the lungs that can be used for multiple clinical applications, potentially including aerosol vaccination. The results of the study will be published online ahead of print in the December issue of Med (10.1016/j.medj.2020.10.005).
Targeted pulmonary delivery may have many conceptual advantages over other routes of vaccine administration and therapeutics, particularly for certain respiratory infections (including but not limited to SARS-CoV-2) because they arrive directly at the site of the infection. In this study, investigators identified a small protein sequence (peptide) that they could incorporate onto the surface of small, benign virus particles (bacteriophage or phage) so that they can interact with lining cells of the lung. This interaction mediates the transport of the phage across the lung tissue into the systemic circulation. Once in the bloodstream, the engineered phage particles can activate a systemic immune response and the production of specific antibodies against any pathogen such as, for example, SARS-CoV-2.
Peptide-displaying phage particles were introduced via aerosol into the lungs of experimental animal models. They bound to specific cell surface receptors expressed on the lung cells. This specific interaction between ligand and receptor led to the transport of the phage particles across the lung tissue into the systemic circulation. This transport through the lungs occurs via a physiologic pathway that preserves normal respiratory function and homeostasis, which is key in the design of any pulmonary delivery system.
To investigate whether the aerosol administration of phage particles was safe in mice, the team closely evaluated the morphology and physiology of the lungs and compared them with animal models that received only saline or control untargeted phage particles. No pulmonary tissue damage was observed in any of the conditions or any of the models tested. Importantly, phage particles have already been safely used in clinics for almost a century to treat patients with bacterial infections, so they are safe and approved for use in humans.
“Our targeted method of pulmonary delivery is the initial step towards the development of aerosol phage-based vaccines for human applications against multiple diseases,” shares co-corresponding author Renata Pasqualini, PhD, a resident member of Rutgers Cancer Institute and chief of the Division of Cancer Biology, Department of Radiation Oncology at Rutgers New Jersey Medical School. “Other advantages of phage particles are that they are highly stable under harsh environmental conditions, and their large-scale production is extremely cost-effective compared with other vaccine strategies because they don’t need to be kept frozen in a strict cold-chain, which is a particularly important requirement for the developing world.”
“Targeted pulmonary delivery is needle-free and minimally invasive, an attribute particularly relevant in the administration of multi-dose vaccines or other molecules. Also, because the lungs are constantly being exposed to pathogens from the air, they have a high level of immune defense activity, and therefore may represent an efficient site for complete immune protection against airborne pathogens,” adds co-corresponding author Wadih Arap, MD, PhD, who is the director of Rutgers Cancer Institute of New Jersey at University Hospital and chief of the Division of Hematology/Oncology at Rutgers New Jersey Medical School.
Phage-based vaccines trigger a sustained and protective immune response, without any detectable toxic effects in the experimental models tested. Phage particles can be genetically engineered to develop different types of vaccines against other infectious pathogens, as well as other types of medicines which could yield other targeted aerosol applications.
“The value of this approach comes from the exquisite specificity of the interaction between the targeted phage particles and the lung cells. This enables more effective delivery of therapeutics or vaccines while reducing the chance of toxic side effects,” notes co-first author Daniela I. Staquicini, PhD, a researcher at Rutgers Cancer Institute of New Jersey at University Hospital and Rutgers New Jersey Medical School. “We hope that this work will play a crucial role in the development of vaccines and treatments to block the spread of pulmonary diseases, possibly already for the current coronavirus global pandemic, especially for underserved populations.”
Along with Drs. Pasqualini, Arap and Staquicini, the other authors on the paper are: E. Magda Barbu, The University of TexasM. D. Anderson Cancer Center; Rachel L. Zemans, University of Michigan Health System; Beth K. Dray, The University of TexasM. D. Anderson Cancer Center; Prashant Dogra, Houston Methodist Research Institute; Marina Cardó-Vila and Cindy K. Miranti, The University of Arizona Cancer Center; Wallace B. Baze, The University of TexasM. D. Anderson Cancer Center; Luisa L. Villa and Jorge Kalil, University of São Paulo Medical School; Geetanjali Sharma and Eric R. Prossnitz, University of New Mexico Comprehensive Cancer Center; Zhihui Wang, Houston Methodist Research Institute; Vittorio Cristini, Houston Methodist Research Institute and the University of TexasM.D. Anderson Cancer Center; Richard L. Sidman, Harvard Medical School; Andrew R. Berman, Rutgers New Jersey Medical School; Reynold A. Panettieri Jr.,Rutgers Institute for Translational Medicine and Science; and Rubin M. Tuder,University of Colorado Anschutz Medical Campus.
This work was supported in part by core services from National Cancer Institute Cancer Center Support Grants to the University of Texas M.D. Anderson Cancer Center (CA016672), University of New Mexico Comprehensive Cancer Center (CA118100), Rutgers Cancer Institute of New Jersey (CA072720), and by research awards from the Gillson-Longenbaugh Foundation (to Pasqualini and Arap). Author disclosures and other information can be found here.
About Rutgers Cancer Institute of New Jersey
As New Jersey’s only National Cancer Institute-designated Comprehensive Cancer Center, Rutgers Cancer Institute, together with RWJBarnabas Health, offers the most advanced cancer treatment options including bone marrow transplantation, proton therapy, CAR T-cell therapy and complex surgical procedures. Along with clinical trials and novel therapeutics such as precision medicine and immunotherapy – many of which are not widely available – patients have access to these cutting-edge therapies at Rutgers Cancer Institute of New Jersey in New Brunswick, Rutgers Cancer Institute of New Jersey at University Hospital in Newark, as well as through RWJBarnabas Health facilities. To make a tax-deductible gift to support Rutgers Cancer Institute of New Jersey, call 848-932-8013 or visit www.cinj.org/giving.
###
For journalists – contact:
Krista Didzbalis
Media Relations Assistant
908-812-6114
krista.didzbalis@rutgers.edu
For patient appointments/inquiries – contact:
844-CANCERNJ (844-226-2376)

