Our Research
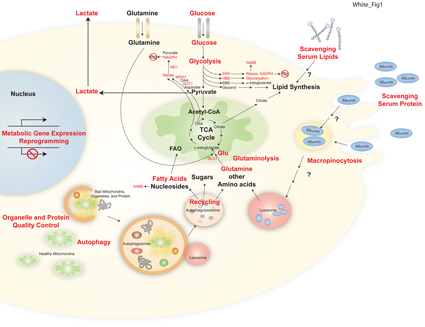
Current research of the White Laboratory at Rutgers Cancer Institute has focused on translational research modulating the apoptosis pathway for cancer therapy and on the role of autophagy and cellular metabolism in cancer progression and treatment. The White group discovered that tumor cells activate the cellular self-cannibalization process of autophagy to survive the stress of tumor growth. This was the first demonstration that autophagy is a cancer survival mechanism for solid tumors and that inhibition of autophagy may be a novel approach to improve solid tumor therapy.
In collaboration with the laboratory of Dr. Josh Rabinowitz at Princeton University the White group went on to demonstrate that tumor cells require autophagy to sustain tumor metabolism and survival. In Ras-driven cancers, autophagy is induced and critical for tumor growth. In models of Ras-driven lung cancer, autophagy is required for progression to aggressive carcinomas, as autophagy blockade causes lung tumors instead to progress to benign oncocytomas. Thus, autophagy promotes the growth and progression of lung cancer to aggressive disease, and autophagy inhibition is a novel means to suppress lung tumor growth and divert progression to benign disease. Clinical trials to test this concept are underway.
Projects
Ludwig Institute for Cancer Research
Award # N/A
PI: White, E.
Research spanning metabolic interaction between host and tumor, dietary modulation of cancer and therapy, and metabolic regulation of the anti-cancer immune response. Dr. White is Associate Director of the Ludwig Princeton Branch at Princeton University. Ludwigcancer.princeton.edu
National Cancer Institute
Impact of Mutation Burden on Cancer Growth and the Immune Landscape
Award #: NIH 5R01CA243547
MPI: White, E., Lattime, E., Ganesan, S.
The goal of this project is to model increased tumor mutation burden in mice to examine the impact on the anti-tumor immune response and response to immune checkpoint blockade. Dr. White is responsible for developing the mouse models for cancer with altered mutation burden in the nuclear and mitochondrial genomes due to proofreading mutations in polymerase epsilon, delta1 and gamma.
National Cancer Institute
Tumor Cell Dependence on Host Metabolism
Award # 5R01CA163591
MPI: White, E., Rabinowitz, J. D.
This project is to define how circulating nutrients promote tumor metabolism, to determine how host autophagy promotes tumor growth, and how dietary modulation can alter metabolism for cancer therapy.
Cancer Cachexia Action Network (Cancer Research UK)
Award # CGCSDF-2021/100003
National Cancer Institute
Award # 1OT2CA278609-01
The CANcer Cachexia Action Network: Cancer Grand Challenges Team CANCAN
Lead PI: White, E.
The major goals of this project are to lead a basic, translational and clinical research team to determine the mechanisms underlying cancer cachexia and to develop therapeutics and clinical centers with which to eradicate it.
Fifth Generation, Inc.
Fasting and Cancer Prevention, Growth, and Treatment
Award # N/A
PI: White, E.
To determine the role of fasting in cancer prevention using pre-clinical cancer models.
Cancer Center Support Grant
Award # P30 CA072720
PI: Libutti, S. J.
This grant provides an organizational focus and stimulus for the highest quality cancer research that effectively promotes interdisciplinary scientific research, and to take maximum collective advantage of scientific opportunities and institutional resources aimed toward the ultimate goal of reducing cancer incidence, morbidity and mortality. Funds from this grant support Rutgers Cancer Institute Cores and Program meetings. Dr. White is Chief Scientific Officer and Deputy Director for the Rutgers Cancer Institute.